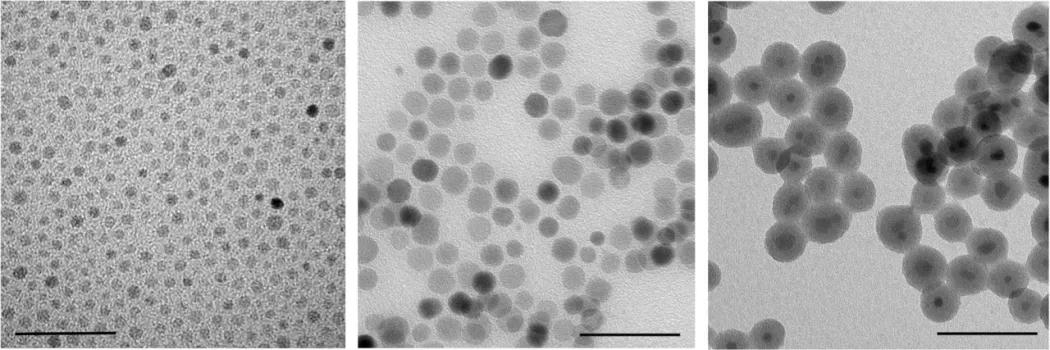
Nanomaterials based on magnetic oxides have formed an important part of our research almost for ten years. Major efforts have been directed towards finding novel methods for the synthesis of magnetic nanoparticles with precisely controlled magnetic properties and for advanced modification of their surface, including organic functionalization. At the same time, their magnetism and structural peculiarities have been thoroughly studied and compared to bulk samples based on SQUID magnetometry, x-ray and neutron diffraction measurements, Mössbauer spectroscopy, electron microscopy, and determination of oxygen stoichiometry. Principal studies have been focussed on monodisperse ferrite nanoparticles and nanocrystalline phases of ferromagnetic manganites La1-xSrxMnO3.
Apart from fundamental physical issues, specific properties important for applications of magnetic nanoparticles have been systematically analysed. Our efforts have been primarily concentrated on applications of magnetic nanoparticles in medicine and biological research and the latter direction has prevailed recently. Hence, we have developed magnetic nanoparticles for applications in MRI (magnetic resonance imaging) and dual imaging [1], magnetic separation [2], and magnetically induced hyperthermia (treatment of tumors by heat produced by suitable magnetic agents in an AC field) [3]. In addition, we have started to develop magnetic nanoparticles for specific applications in analytical and bioanalytical chemistry.
We have been dealing with nanoparticles based mostly on ferrite or manganites cores, i.e., binary or ternary oxides whose magnetic properties can be easily adjusted by varying their composition [4]. In this regard, two examples should be mentioned. First, the Curie temperature of the La1-xSrxMnO3 manganite within the ferromagnetic region (0.21-xSrxMnO3 phase, the Curie temperature is somewhat lower due to finite size effects. Importantly, the Curie point can be adjusted to the value slightly above the body temperature, which enables the self-controlled heating mechanism of La1-xSrxMnO3 nanoparticles in magnetically induced hyperthermia. Second, the magnetization of the ferrimagnetic Co1-xZnxFe2O4 phase reaches a maximum for certain composition since the magnetization of ferrite is given by antiferromagnetic coupling between the two sublattices and the diamagnetic Zn2+ decreases primarily the magnetic moment of one of them. Thus, the magnetization of Co-ferrite can be further increased through doping with zinc. Importantly, a high magnetization of magnetic nanoparticles is crucial for the development of efficient contrast and labeling agents for MRI. In conclusion, binary, ternary, or even more complex oxides offer clear advantages in comparison to simple iron oxides, like magnetite or maghemite.
However, the development of magnetic nanoparticles for any biological or even analytical application requires to build a barrier between the magnetic core and the surrounding environment. Such barrier has to prevent any harmful chemical effects of the cores on a biological system, provide the cores with colloidal stability in aqueous suspension, and enable specific covalent functionalization for advanced applications. Following these aims, we have applied various procedures whereby magnetic cores were encapsulated into simple and durable shells or elaborate coating composed of several layers. These were formed by silica, organically modified silica, including layers covalently tagged with fluorescent molecules, but also mPEG/PEG (methoxypoly(ethylene glycole)/poly(ethylene glycole)) chains, and gold nanoshells.
Detailed physical studies addressed, for example, the Mn3+/Mn4+ ordering in „half-doped“ systems Pr0.50Ca0.50MnO3 and La0.50Ca0.50MnO3 and they showed that the ordering is suppressed in nanocrystallites [5]. Further, the study of nanocrystalline La1-xSrxMnO3 phases indicated the crucial role of the surface of nanoparticles in the actual oxygen stoichiometry of these phases, which significantly affects their magnetism and structure [6]. The saturation of the surface with oxygen atoms leads to higher oxidation state of manganese in several surface layers and thus to a compressive strain. Similar effects were revealed in manganite nanoparticles prepared in flux [7]. However, in this particular case the concentration gradient of Sr/La within a single particle seems to contribute as well. Ferrite nanoparticles were subjected to two types of fundamental studies. The first type of studies aimed at the structure and magnetic behaviour of individual particles and the second type of studies was devoted to collective phenomena. For example, the cation distribution in Co-Zn ferrite was analysed and proved to be different compared to bulk samples [8], whereas monodisperse magnetic nanoparticles of zinc-doped magnetite provided an excellent model to analyse the super spin glass transition [9].
The application studies were particularly extensive owing to our strong collaboration with several biological and biomedical laboratories, namely with the Faculty of Science of the Charles University (Department of Cell biology), the Institute of Clinical and Experimental Medicine, and the Institute of Experimental Medicine AS CR. Three topics were focused: the transverse relaxivity of nanoparticles and their use as contrast and labelling agents in MRI, heating effects of magnetic nanoparticles in AC field and potential application in magnetically induced hyperthermia, and finally biological properties of nanoparticles.
- Trachtová, Š., Kaman, O., Španová, A., Veverka, P., Pollert, E., and Rittich, B. 2011. Silica-coated La0.75Sr0.25MnO3 nanoparticles for magnetically driven DNA isolation. Journal of Separation Science, 34(21): 3077-3082.
- Kaman, O., Veverka, P., Jirák, Z., Maryško, M., Knížek, K., Veverka, M., Kašpar, P., Burian, M., Šepelák, V., and Pollert, E. 2011. The magnetic and hyperthermia studies of bare and silica-coated La0.75Sr0.25MnO3 nanoparticles. Journal of Nanoparticle Research, 13(3): 1237-1252.
- Pollert, E., Veverka, P., Veverka, M., Kaman, O., Závěta, K., Vasseur, S., Epherre, R., Goglio, G., and Duguet, E. 2009. Search of new core materials for magnetic fluid hyperthermia: Preliminary chemical and physical issues. Progress in Solid State Chemistry, 37(1): 1-14.
- Jirák, Z., Hadová, E., Kaman, O., Knížek, K., Maryško, M., Pollert, E., Dlouhá, M., and Vratislav, S. 2010. Ferromagnetism versus charge ordering in the Pr0.5Ca0.5MnO3 and La0.5Ca0.5MnO3 nanocrystals. Physical Review B, 81(2): art. no. 024403.
- Žvátora, P., Veverka, M., Veverka, P., Knížek, K., Závěta, K., Pollert, E., Král, V., Goglio, G., Duguet, E., and Kaman, O. 2013. Influence of surface and finite size effects on the structural and magnetic properties of nanocrystalline lanthanum strontium perovskite manganites. Journal of Solid State Chemistry, 204: 373-379.
- Kačenka, M., Kaman, O., Jirák, Z., Maryško, M., Veverka, P., Veverka, M., and Vratislav, S. 2015. The magnetic and neutron diffraction studies of La1−xSrxMnO3 nanoparticles prepared via molten salt synthesis. Journal of Solid State Chemistry, 221(0): 364-372.
- Veverka, M., Jirák, Z., Kaman, O., Knížek, K., Maryško, M., Pollert, E., Závěta, K., Lančok, A., Dlouhá, M., and Vratislav, S. 2011. Distribution of cations in nanosize and bulk Co-Zn ferrites. Nanotechnology, 22(34): art. no. 345701.
- Kaman, O., Kořínková, T., Jirák, Z., Maryško, M., and Veverka, M. 2015. The superspin glass transition in zinc ferrite nanoparticles. Journal of Applied Physics, 117: art. no. 17C706.
- Kačenka, M., Kaman, O., Kotek, J., Falteisek, L., Černý, J., Jirák, D., Herynek, V., Zacharovová, K., Berková, Z., Jendelová, P., Kupčík, J., Pollert, E., Veverka, P., and Lukeš, I. 2011. Dual imaging probes for magnetic resonance imaging and fluorescence microscopy based on perovskite manganite nanoparticles. Journal of Materials Chemistry, 21(1): 157-164.